

Also replace URL for the actual url of this page (The stay, ok?). This distance between an atoms nucleus and outer electron shell. Now replace dd, mmmm and yyyy with the day, month, and year you browsed this page. "Atomic Radius of Calcium (Ca) [& State, Uses, Discovery. To make your life (and citation) easier just copy & paste the information below into your assignment or essay: That gives credibility to your paper and it is sometimes required in higher education. When you need to include a fact or piece of information in an assignment or essay you should also include where and how you found that piece of information. How about an incentive to share this post? (You will help other colleagues find this blog)ĭownload and enjoy this complete and colored periodic table for you to edit and enjoy. Need an editable periodic table to edit? Maybe add your school logo, work team or anything else to make your paper look cool?Īlong with basic atom / element information (like Calcium atomic radius and all the other atomic data), it also comes with color coded info about: State (Gas, Liquid or Solid at room temperature), Groups/series details and much more. Its corresponding cation, Ca2+, has an ionic radius of 99pm. How a small number of atoms can be joined and form completely different substances Chad breaks down the trend for Atomic and Ionic Radius also discussing Effective Nuclear.Want to learn more details and data about Calcium (Ca)? Check my Elements Comprehensive List.Īre you having trouble understanding the basics of atomic elements? This video will walk you through: Fifth most abundant element in the earth's crust (41,500 ppm).
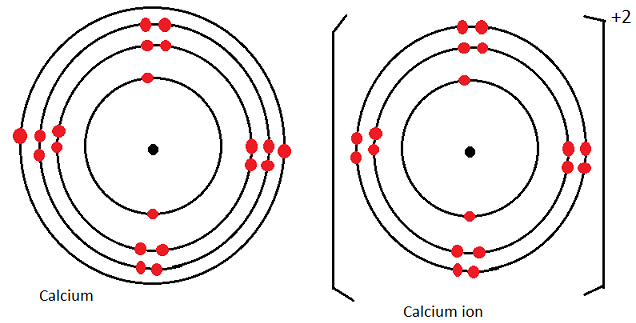
Pure metal is produced by replacing the calcium in lime (calcium carbonate, CaCO3) with aluminium in hot, low pressure retorts.įairly hard, silvery-white metal. Obtained from minerals like chalk, limestone & marble. Virtually no use for the pure metal, however two of its compounds are, lime (CaO) and gypsum (CaSO4), are in great demand by a number of industries. Used by many forms of life to make shells and bones. In the case of Calcium the atomic radius is 2.23 Å. There are cool facts about Calcium that most don't know about. Note: Learn more about the atomic radius here. Ok, so what is the atomic radius of an atom of Ca? All atoms have a (theoretical) atomic radius, even Calcium.


 0 kommentar(er)
0 kommentar(er)
